You might want to start with our general overview of biologics and biosimilars in Canada, which includes basic information and a list of questions to ask your health care providers. You can also obtain the information as a printable PDF or the Gastrointestinal Society can mail you pamphlets.
We use large molecule biologic medicines in the treatment of chronic inflammatory conditions where often the exact cause (or aetiology) of disease is unknown.1 We do know, however, that many factors can drive chronic inflammation, including activated immune cells, cellular processes, and cell signalling molecules (cytokines), and that inflammation is a dynamic process.2 As a result, the factors, such as specific cytokines, driving chronic inflammation in one individual may vary when compared to another individual with the same chronic illness or at different stages of disease. This explains, to some extent, why a single biologic medicine that results in resolution of inflammation in one patient, may not work for another patient with the same disease.1,3 It also highlights the need for the continued development of new and innovative targets in the biologics treatment space, as our understanding of the different factors driving inflammation in chronic inflammatory diseases increases.
One of the first large molecule biologic medicines developed and used widely in the treatment of chronic inflammatory diseases targets one such inflammatory cytokine: tumor necrosis factor (TNF)-a.4 This large biologic medicine was named infliximab (originator brand name: Remicade®) and is an antibody that binds to and sequesters TNF-a, blocking it from driving inflammatory responses. Of the large molecule biologic medicines currently approved in Canada to treat inflammatory diseases, five of them work by targeting and blocking TNF-a through different mechanisms.1,3 The remaining seven target other inflammatory cytokines, such as interleukin (IL) 12 and 23, bind cytokine receptors to block their activation, or block immune cells from being recruited to the site of inflammation (Table 1).
| Molecule | Originator Biologic | Target |
| abatacept | Orencia® | CD80/86 (T cells – type of immune cell) |
| adalimumab | Humira® | TNF-a (cytokine) |
| anakinra | Kineret® | IL-1 Receptor (cytokine receptor) |
| certolizumab pegol | Cimzia® | TNF-a (cytokine) |
| etanercept | Enbrel® | TNF-a (cytokine) |
| golimumab | Simponi® | TNF-a (cytokine) |
| infliximab | Remicade® | TNF-a (cytokine) |
| rituximab | Rituxan® | CD20 (B cells – type of immune cell) |
| secukinumab | Cosentyx® | IL-17A (cytokine) |
| tocilizumab | ActemraTM | IL-6 Receptor (cytokine receptor) |
| ustekinumab | Stelara® | IL-12/Il-23 (cytokine) |
| vedolizumab | Entyvio® | a4b7 (T cells) |
Table 1.1 Targets of large molecule biologic medicines used in the treatment of chronic inflammatory diseases. Given their ability to block specific immune signalling pathways and cell types, the use of biologic medications in the treatment of chronic inflammatory diseases comes with many potential side effects and safety risks that should be discussed with a medical professional prior to administration.
*Note: different molecules targeting the same cytokine or receptors (e.g. TNF-a) bind and inactivate the target through different mechanisms.
The illustrations below depict how two originator biologics targeting TNF-a, along with their biosimilars, exert their effects in inflamed joint or intestinal tissue as observed in rheumatoid arthritis and inflammatory bowel diseases, respectively.
Biologics in Rheumatoid Arthritis
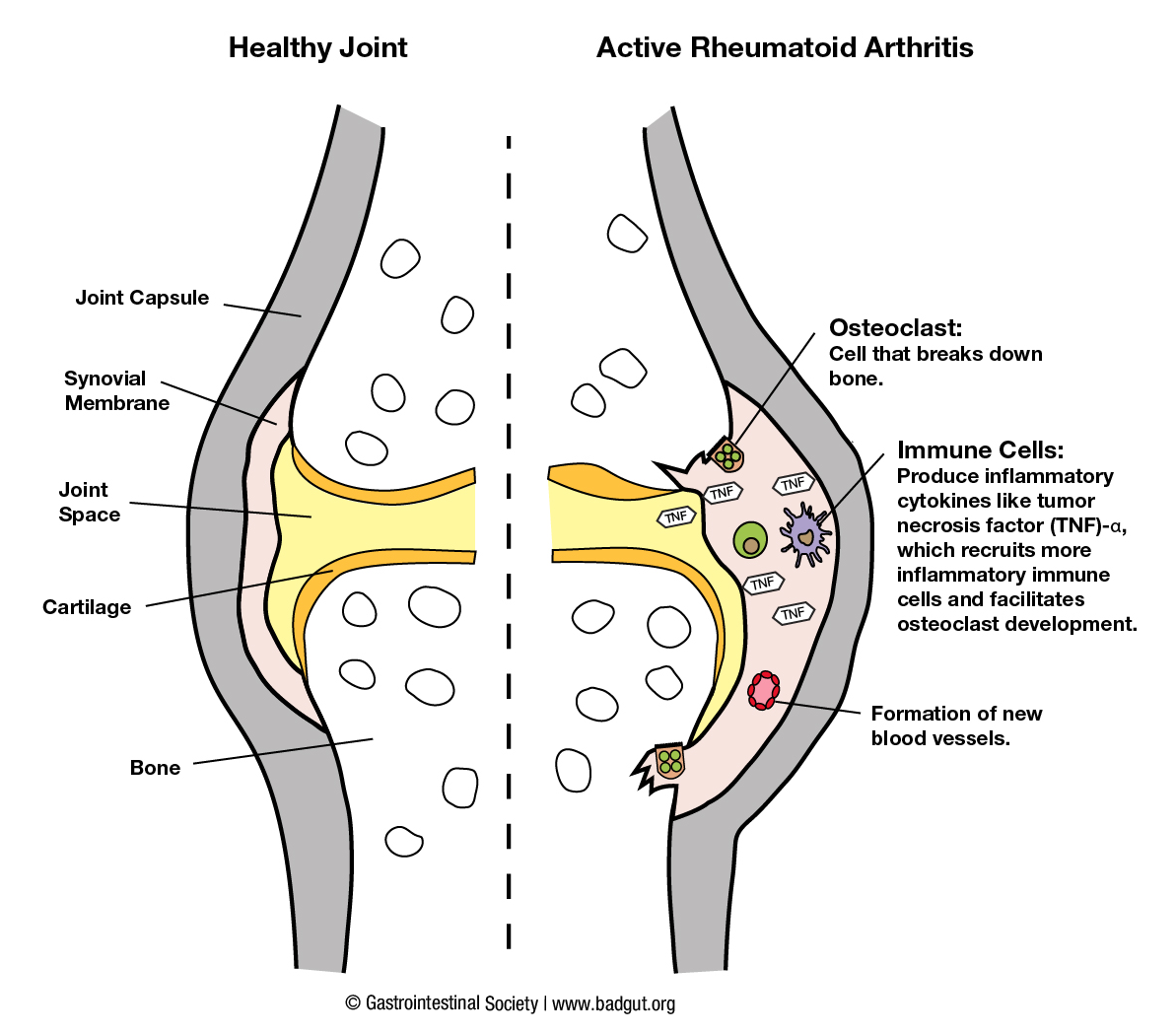
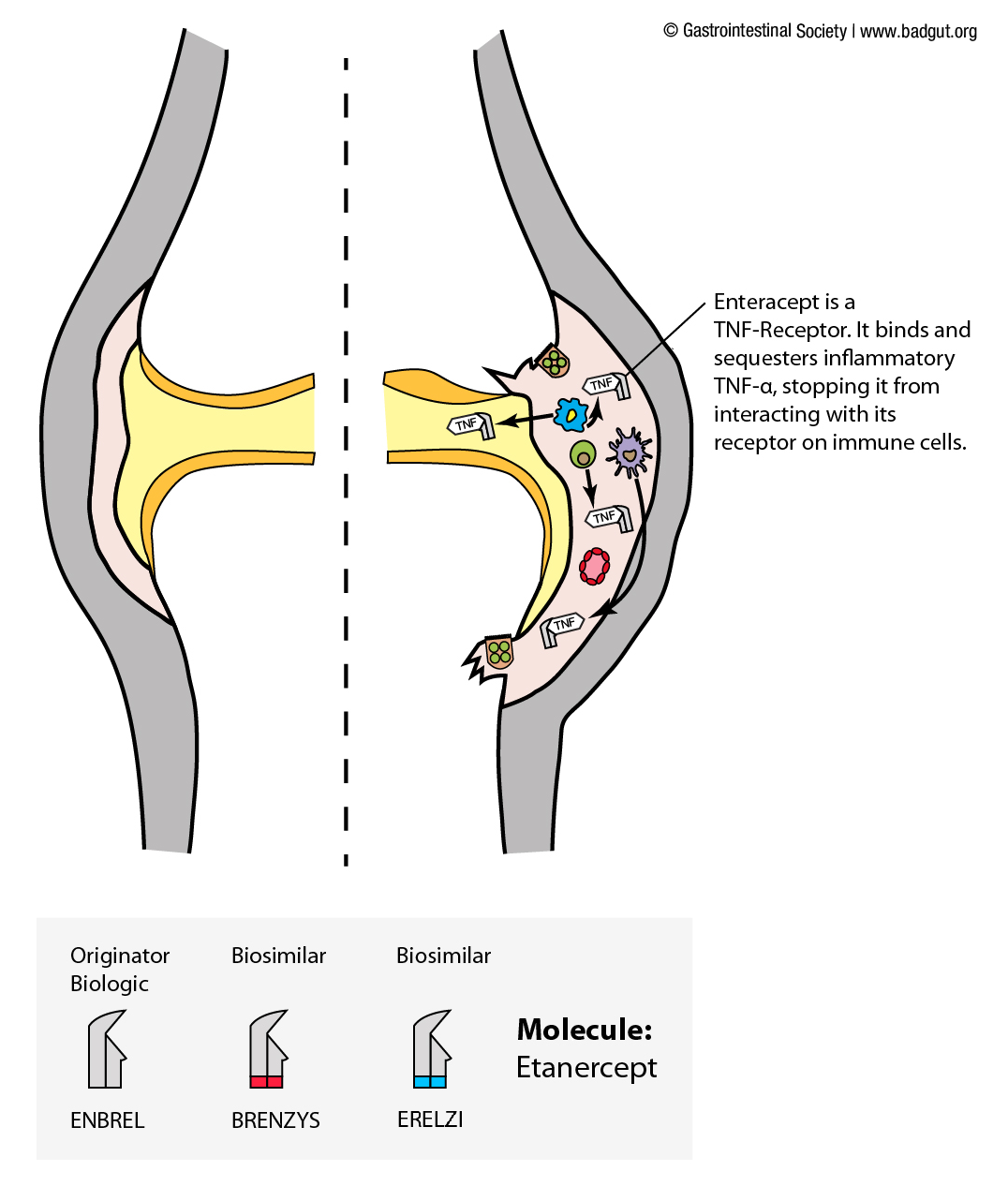
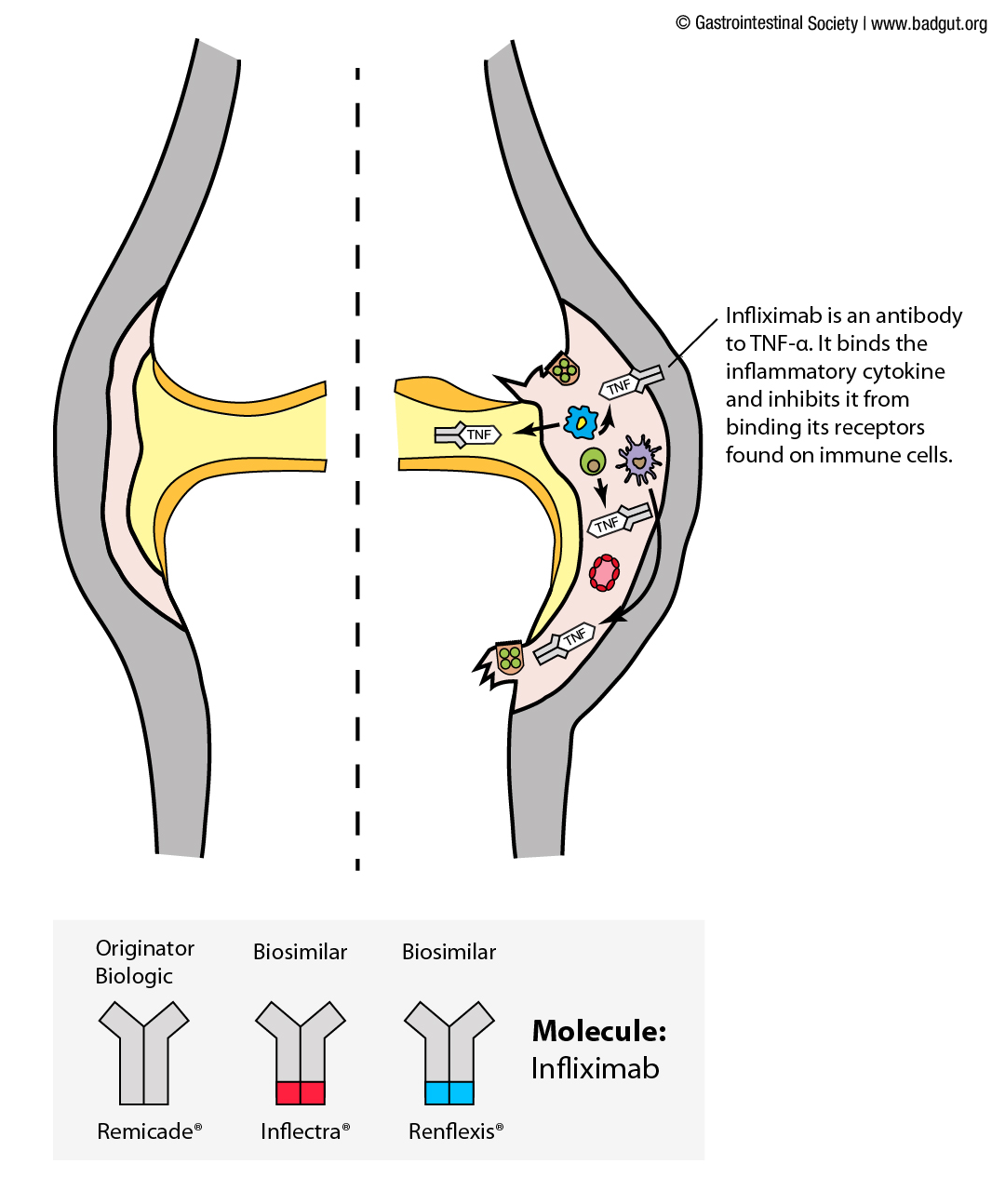
Legend for joint inflammation diagrams (1):
In a healthy joint, the synovial membrane, which lines the non-weight bearing surface of the joint, produces fluid that is lubricating and nourishing.5 In contrast, in an inflamed joint the synovial membrane is filled with immune cells (e.g. T cells and other chronic inflammatory cells) and the cells within the membrane itself begin to divide (proliferate).5 The immune cells within the inflamed joint produce many pro-inflammatory cytokines (e.g. TNF-a, IL-17A) that facilitate further immune cell recruitment to the membrane, production of cytokines, and proliferation of the membrane. If left uncontrolled, the disease progresses, and the proliferating synovial membrane cells can become invasive and begin to break down cartilage and bone at the joint.6
Among chronic inflammatory conditions, infliximab was first used in the treatment of rheumatoid arthritis.4 It is administered intravenously and disperses throughout the body, demonstrated further in Figure (2). Biologic medications cannot be taken orally as digestive enzymes found in the gastrointestinal tract would break them down before the body could absorb them. A second biologic medicine targeting TNF-a that we use in the treatment of rheumatoid arthritis is etanercept. In comparison to infliximab, which is an antibody to TNF-a that binds and sequesters it, etanercept acts more like a decoy receptor for TNF-a.7 The end goal of both infliximab and etanercept is to block signalling of TNF-a in the body. Etanercept is administered by injection under the skin (subcutaneously), and the small blood vessels in this area absorb it, then it enters the circulatory system and can exert its effects throughout the body.
Biologics in Inflammatory Bowel Disease
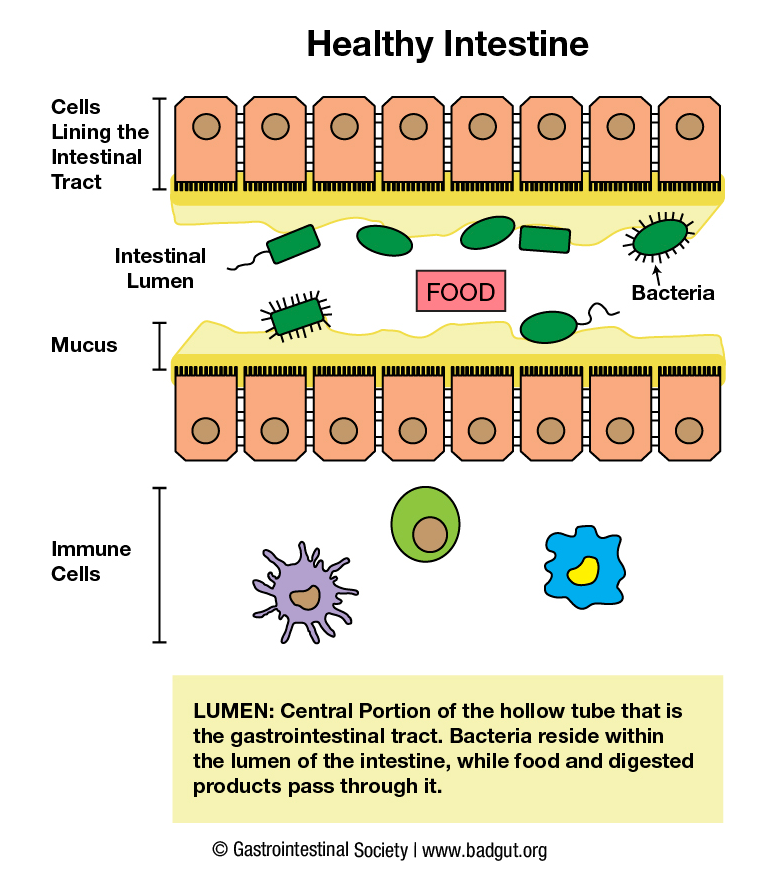
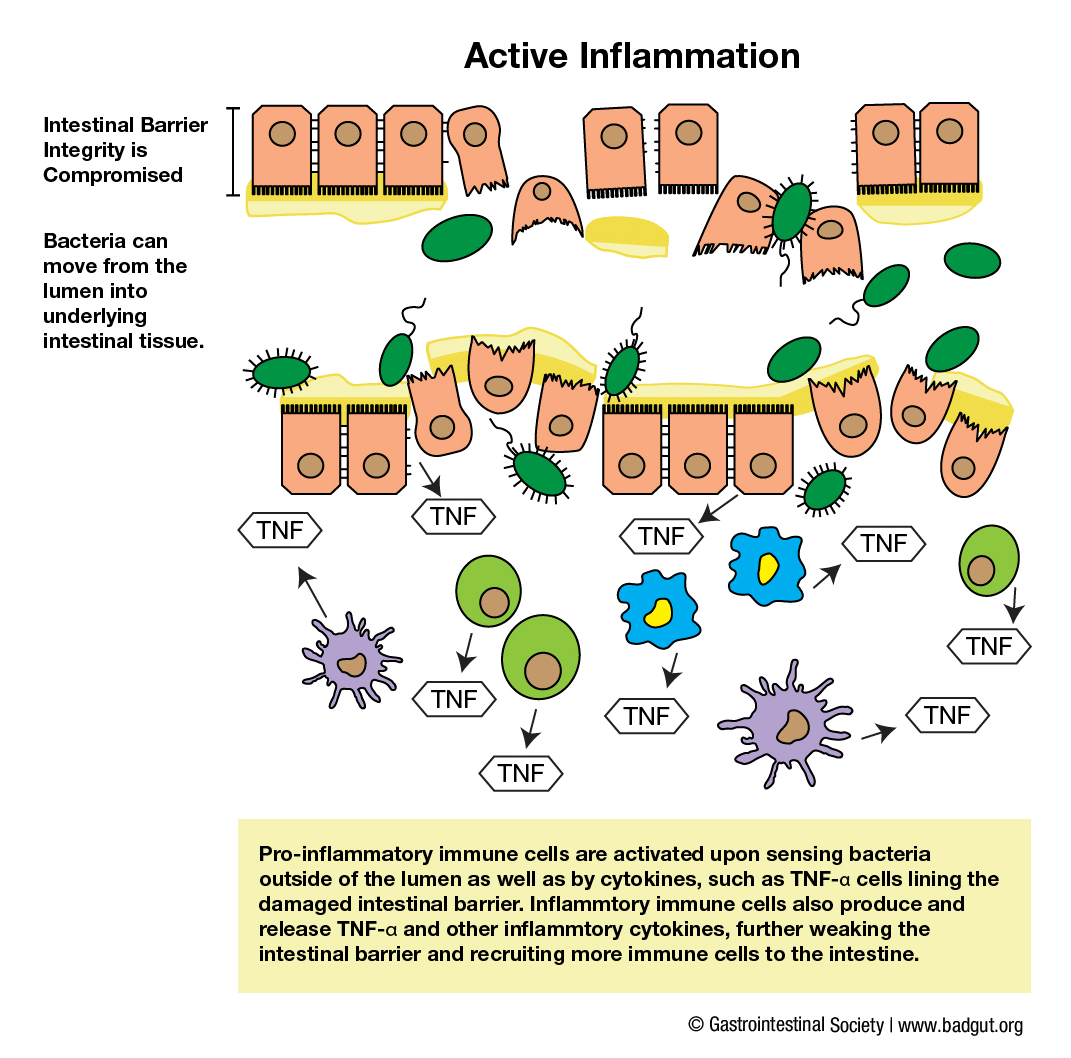
Legend for intestinal inflammation diagrams (2):
In a healthy intestine, a zone of separation exists between the bacteria residing in the central portion of the intestinal tract (lumen), the single cell layer intestinal lining, and underlying tissue and immune cells.8 A mucus layer and antimicrobial proteins released by the single cell layer and immune cells facilitate separation.9
When inflammatory conditions disrupt this balance, pro-inflammatory immune cells are activated upon sensing bacteria outside of the lumen as well as inflammatory cytokines, such as TNF-a, released by cells lining the damaged intestinal barrier and underlying immune cells.8,9 Release of inflammatory cytokines further activates and recruits more immune cells to the intestine, which in turn release more TNF-a, resulting in too much of this cytokine in the tissue. If left unmanaged, this process can propagate inflammation and result in further damage to the intestinal barrier.
Intravenous infusion of infliximab is one potential treatment that may be effective in managing intestinal inflammation.7 With this route of administration, the medication is delivered directly into a vein, immediately enters the circulatory system of the individual, and is able to exert its potential effects throughout the body. In intestinal tissues where excess TNF-a is a major driver of inflammation, infliximab is able to bind and sequester this inflammatory cytokine when it enters the intestinal tissue from the circulatory system. Infliximab-bound TNF-a is no longer able to exert its pro-inflammatory effects, resulting in a return towards a less inflammatory state and strengthening of the intestinal barrier in those that respond to the medication.
Written by Dr. Ganive Bhinder, BSc, PhD
- Rodgers, K.R. & Chou, R.C. Therapeutic monoclonal antibodies and derivatives: historical perspectives and future directions. Biotechnology Advances. 2016(24):1149-1158
- Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nature Reviews Immunology. 2002(2):364-371
- Strand, V. et al. Biologic therapies in rheumatology: lessons learned, future directions. Nature Reviews Drug Discovery. 2007(6):75-92
- Elliot, M.J. et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor a (cA2) versus placebo in rheumatoid arthritis. The Lancet. 1994(344):1105-1110
- Smolen, J.S. & Steiner, G. Therapeutic strategies for rheumatoid arthritis. Nature Reviews Drug Discovery. 2003(2):473-488
- Korb-Pap, A. et al. Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Annals of the Rheumatic Diseases. 2012(71):1004-1011
- Levin, A.D., et al. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. Journal of Crohn’s and Colitis. 2016:989-997
- Belkaid, Y. & Artis, D. Immunity at the barriers. European Journal of Immunology. 2013(43):3096-3097
- Peterson, L.W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nature Reviews Immunology. 2014(14):141-153